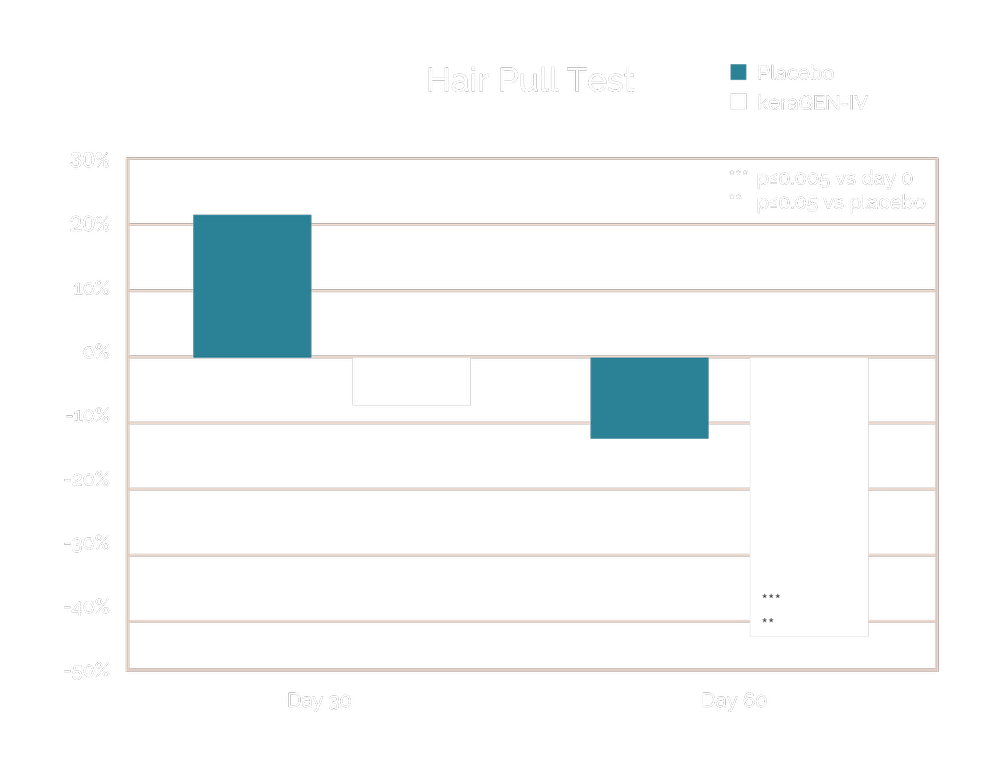
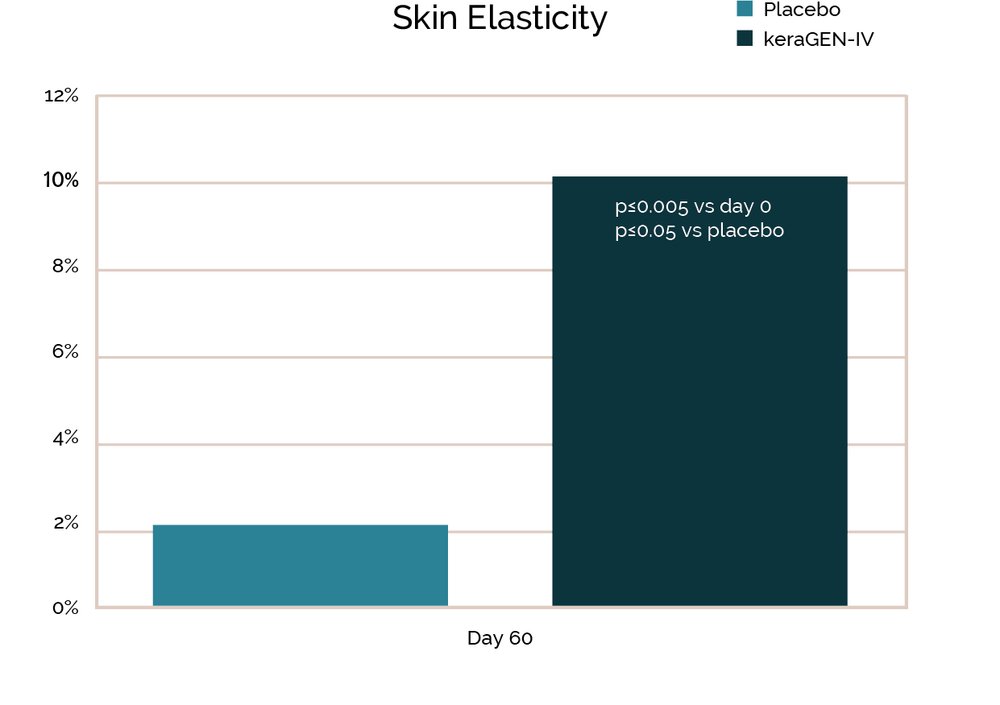
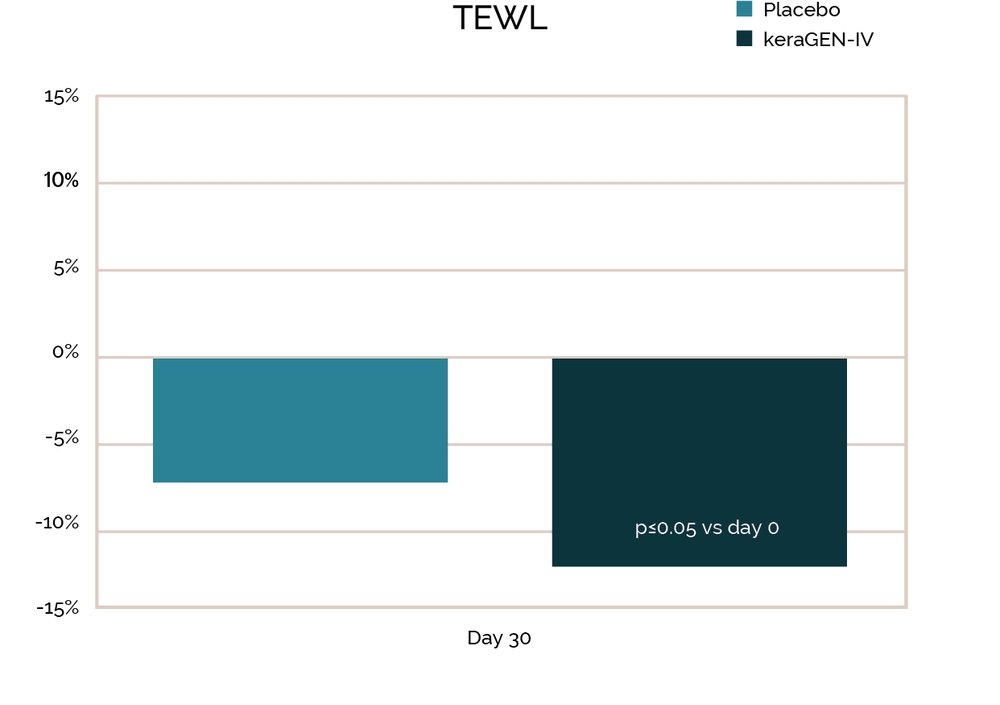
Prior work on keraGEN-IV® identified using in vitro studies on human keratinocytes showed that increased collagen IV expression is induced by this keratin protein. The keraGEN-IV® action of inducing collagen IV expression translates to measurable improvement in hair anchoring, specifically a 43.1% reduction in hair loss in women aged 45-60 with stressed or damaged hair.
Volunteers taking the keraGEN-IV® supplement demonstrated a substantial and statistically significant reduction in hair pull scores on day 60. Reduction compared to day 0 was -43.1% (p≤0.005). The gentle pulling action triggering hair loss is typical of that experienced during combing or styling when hair is damaged through chemical treatments, colour, styling or during peri- or post-menopause.